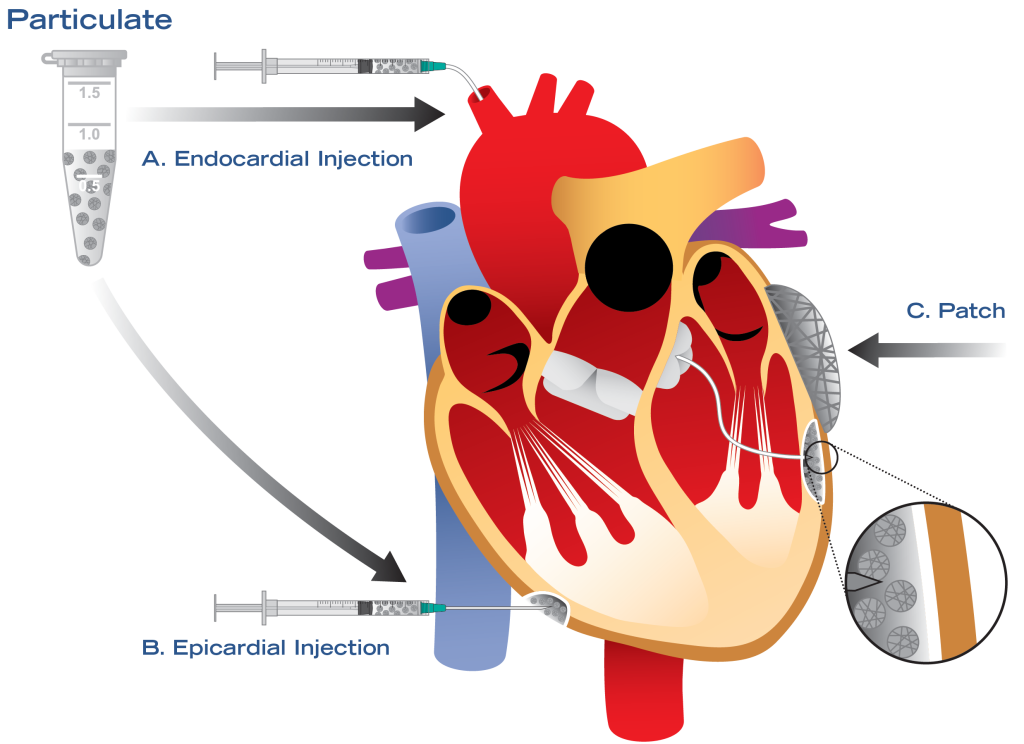
CFX™ is a first-of-its-kind therapeutic biomaterial manufactured using induced pluripotent stem cell technology. Local administration of CFX™ can prevent the pathological changes that lead to heart failure following an acute injury such as myocardial infarction.
Additionally, CFX™, in combination with therapeutic stem cells, can remuscularize scarred myocardium to restore heart function in chronic heart failure.
Cellular Logistics products are exclusively owned by Cellular Logistics and are protected by issued patents in the United States and additional pending patent applications in the United States and foreign jurisdictions.